Mechanical Ventilation:
New strategies could save thousands of lives
In Germany, around 16,000 patients per year require mechanical ventilation for acute respiratory distress syndrome (ARDS). It is additionally called for in severe cases of chronic obstructive pulmonary disease (COPD). In other words, conditions typically associated with smoking. Asthmatics also present an increased risk of lung failure. Other causes of lung failure include allergies and growing resistance to antibiotics, which turns previously treatable conditions such as pneumonia into life-threatening illnesses. The World Health Organization (WHO) estimates that cases of acute pulmonary failure will end up doubling in number between 1990 and 2020.
When we breathe, we inhale vital oxygen as the diaphragm contracts, generating slight negative pressure. Three to five millibar is sufficient for air to flow into our lungs. If the lungs fail, our only chance of survival is immediate artificial ventilation. In contrast to natural respiration, mechanical ventilation uses high pressure. The attending physician only has a few seconds to determine the initial setting of the ventilator using rules of thumb. Here the breathing rate per minute, volume of each breath and positive end-expiratory pressure (PEEP) is used to generate a ventilation model, taking blood levels of oxygen and carbon dioxide as reference points. If the vital oxygen level drops, the doctor turns up the ventilator, with peak pressure of up to 50 millibar not uncommon.
Studies worldwide show that a more gentle ventilation strategy, known as lung-protective ventilation, can significantly improve chances of survival. “Even a pressure of 40 millibar is far too high for the tissue of a diseased lung. Each breath over-expands it, leading to irreversible damage,” explains Prof. Dr. Josef Guttmann, head of the working group for clinical respiratory physiology at the University Medical Center Freiburg, Germany. However, doctors do not yet have a reliable model to guide them. Concerned that too low a pressure might cause the lung to collapse, many doctors prefer to play it safe and select a higher setting. “The logic is flawed,” according to Guttmann, “as every millibar of decreased pressure is worth fighting for to relieve the lungs.”
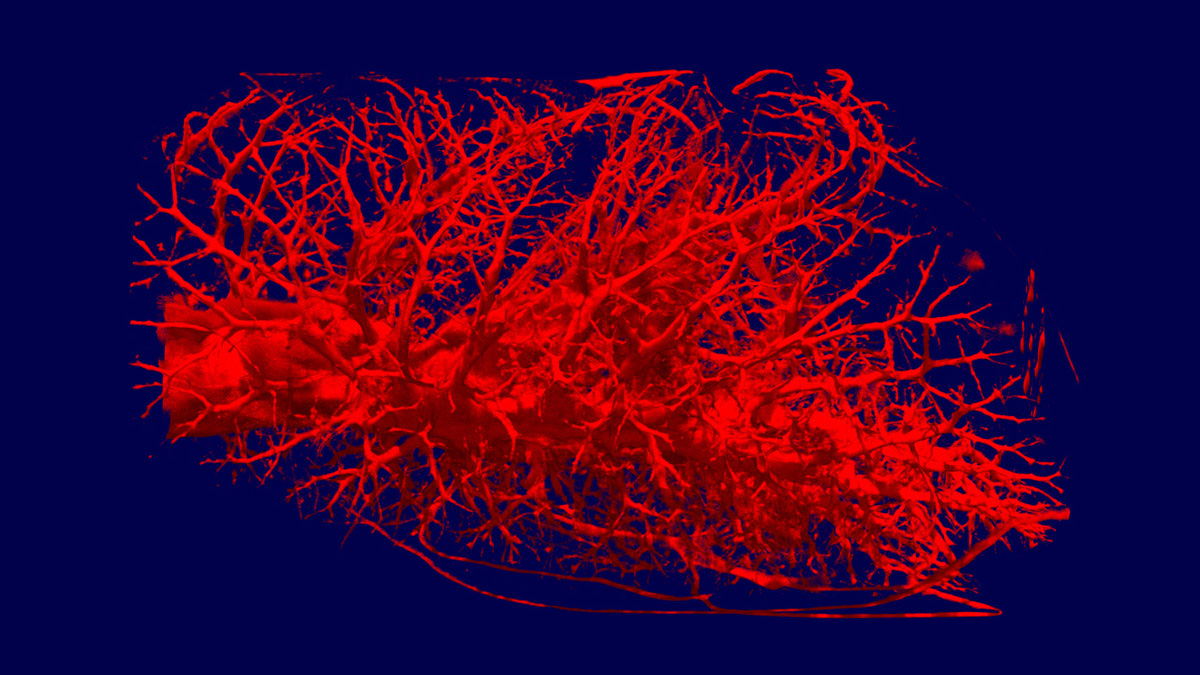
Within the German Research Foundation (DFG) project on protective artificial respiration, Guttmann is now collaborating with scientists from the TUM to develop a model-based strategy for gentle ventilation. Prof. Dr.-Ing. Wolfgang A. Wall, Head of TUM’s Chair of Computational Mechanics (LNM), is working on a computer-aided model of conditions inside the lungs. And Dr. Burkhard Schillinger, of the ANTARES facility at the FRM II neutron source, is supplying the geometric data this simulation requires. Together, they are aiming to establish exactly how the lung’s micromechanics function during breathing. Around 300 million pulmonary alveoli with an internal surface area of approximately 100 square meters enable the exchange of oxygen and carbon dioxide between the airflow and bloodstream. But how does the air reach them? And how does increased pressure effect gas transport and lung tissue?
Textbooks continue to portray the alveoli as tiny cavities or sacs. According to the usual description, when we breathe in, the air flows through the bronchial tubes, which branch off ever more finely until reaching the alveoli, where gas exchange occurs. So based on this model, if the ventilation pressure is increased, more air should flow into the alveoli. In fact, it is rather more complicated than that. The bronchi in a human lung branch into as many as 23 generations. The model calculations show that the air can be transported by convection up to around the 16th generation – but after that, gas exchange would have to take place by diffusion. The researchers are entering completely uncharted territory here.
The finest branches have topologies just a few microns in size. “There is no imaging technique that can show the dynamic processes involved in inhaling and exhaling,” reveals Guttmann. “So we can’t get any further without a dedicated computational model here.” Scientists can identify the structures under a microscope, but dissected tissue samples cannot reproduce the actual conditions inside a functioning lung. And X-ray-based computer tomography does not deliver the desired results either. “X-rays primarily identify heavy atoms,” explains Schillinger. “But spongy lung tissue with its thin walls largely comprises carbon, hydrogen and oxygen atoms, while our finer airways mostly contain oxygen and nitrogen. So that gives us too little contrast to really see much.” The ideal imaging technique for this purpose is neutron tomography. Neutrons “see” light atoms such as hydrogen particularly well. This element is plentiful in lung tissue, but not in the bronchi.
The researchers are now testing their dynamic models on rat lungs using the ANTARES facility at the FRM II neutron source. This facility was specially constructed for neutron tomography and is one of the world’s most powerful. Schillinger needs around 400 to 800 images to obtain a complete 3D scan. Even the FRM II’s high neutron flux still requires an exposure time of approximately 20 seconds for each individual image. “The FRM II is the only device in the world we can use for this kind of research. A lower neutron flux would mean such long exposure times, the tissue would noticeably degrade by the end of the scan,” Schillinger explains. In the facility, the rat lungs are inflated at the ventilation pressure to be examined. An aluminum tube simulates the mechanical support provided by the ribcage. If the researchers adjust the pressure, the capillary geometry changes. This allows them to calculate the elasticity of the lung tissue and identify at what point its limits are reached and damage should be expected.
At the start of the trials, Schillinger was rather skeptical about the idea. “We started with pig lungs, which we bought at the butcher near the FRM II in Garching,” recalls Robert Metzke, responsible for the project at the LNM. “And then, when we saw the first images, we knew we were on the right track.” The scientists are now able to visualize the bronchial branches up to the 12th generation. The only thing holding the method back at the moment is the resolution of the detectors. Neutron tomography uses parallel neutron beams, preventing the enlargement possible with the cone beams of an X-ray tube, for instance. The detector’s resolution mainly depends on its slice thickness. The scientists are now working with purpose-built detectors for this project, which boast a slice thickness of just 50 microns. This enables a resolution of approximately 40 microns – and that’s as far as it goes. While the manufacturers work on even thinner detectors, the scientists are investigating other means of increasing contrast.
“The 3D models at the ANTARES facility are a key resource for us,” confirms Prof. Wall. “They provide us with a reality check, since we obviously can’t measure all this on live patients.” Prof. Guttmann is also enthusiastic about the successful collaboration: “We know that diseased lung tissue has substantially less elasticity than that of a healthy organ. The more we learn about the actual conditions in the lungs, the more gently we can ventilate.” The long-term aim here is the production of self-learning ventilation equipment, building on the scientists’ findings to reduce air pressure to the necessary minimum for each individual patient. This would then avoid the frequently fatal lung damage currently associated with mechanical ventilation.
Links:
Study on protective mechanical ventilation
Imagery:
https://mediatum2.ub.tum.de/node?id=670270&dir=670270
Contact:
Prof. Dipl.-Ing. Dr. Josef Guttmann
University Freiburg
Department Anaesthesiology and Intensive Care
Experimental Anaesthesiology
Hugstetter Str. 55
D-79106 Freiburg
Tel.: +49 761 270 2333
Fax: +49 761 270 2396
E-Mail: josef.guttmann@uniklinik-freiburg.de
Web: http://www.uniklinik-freiburg.de/expanaesthesie/live/index.html
Prof. Dr.-Ing. Wolfgang A. Wall
Technische Universität München
Faculty of Mechanical Engineering
Chair of Computational Mechanics
Boltzmannstr. 15
D-85747 Garching
Tel.: +49 89 289 15300
Fax: +49 89 289 15301
E-Mail: wall@lnm.mw.tum.de
Web: http://www.lnm.mw.tum.de
Dr. Burkhard Schillinger
Technische Universität München
Research Neuron Source Heinz Meier-Leibnitz
Neutron Tomography ANTARES
Lichtenbergstr. 1
D-85747 Garching
Tel.: +49 89 289 12185
Fax: +49 89 289 13776
E-Mail: burkhard.schillinger@frm2.tum.de
Web: http://www.frm2.tum.de