Movable cytoskeleton membrane fabricated for first time
Artificial cells take their first steps
Cells are complex objects with a sophisticated metabolic system. Their evolutionary ancestors, the primordial cells, were merely composed of a membrane and a few molecules. These were minimalistic yet perfectly functioning systems.
Thus, "back to the origins of the cell" became the motto of the group of TUM-Prof. Andreas Bausch, who is member of the cluster of excellence "Nanosystems Initiative Munich (NIM)" and his international partners. Their dream is to create a simple cell model with a specific function using a few basic ingredients. In this sense they are following the principle of synthetic biology in which individual cellular building blocks are assembled to create artificial biological systems with new characteristics.
The vision of the biophysicists was to create a cell-like model with a biomechanical function. It should be able to move and change its shape without external influences. They explain how they achieved this goal in their latest publication in Science.
The magic ball
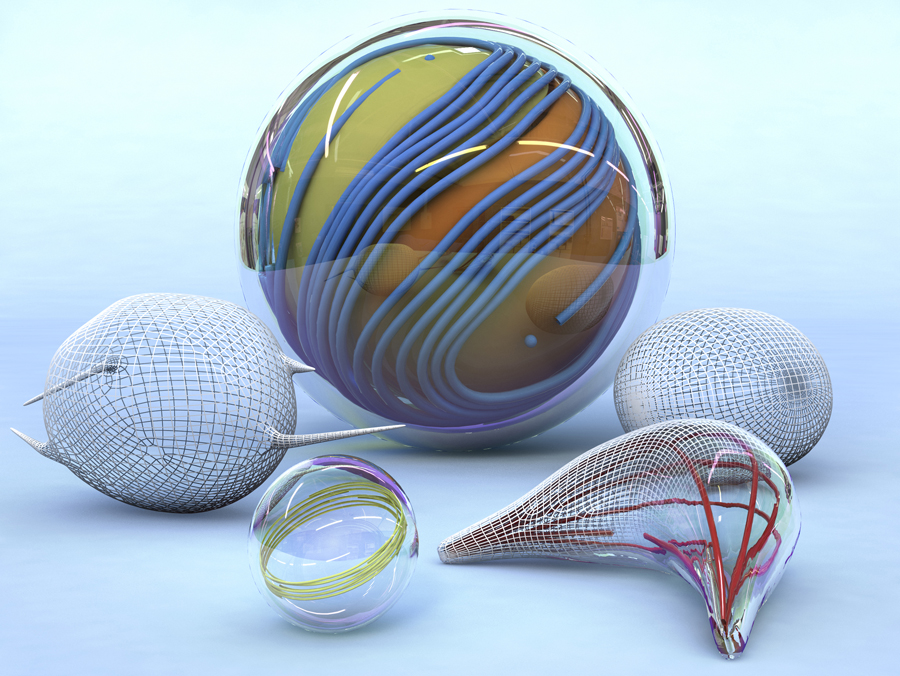
The biophysicists’ model comprises a membrane shell, two different kinds of biomolecules and some kind of fuel. The envelope, also known as a vesicle, is made of a double-layered lipid membrane, analogous of natural cell membranes. The scientists filled the vesicals with microtubules, tube-shaped components of the cytoskeleton, and kinesin molecules. In cells, kinesins normally function as molecular motors that transport cellular building blocks along the microtubules. In the experiment, these motors permanently push the tubules alongside each other. For this, kinesins require the energy carrier ATP, which was also available in the experimental setup.
From a physical perspective, the microtubules form a two-dimensional liquid crystal under the membrane, which is in a permanent state of motion. "One can picture the liquid crystal layer as tree logs drifting on the surface of a lake," explains Felix Keber, lead author of the study. "When it becomes too congested, they line up in parallel but can still drift alongside each other."
Migrating faults
Decisive for the deformation of the artificial cell construction is that, even in its state of rest, the liquid crystal must always contain faults. Mathematicians explain these kinds of phenomena by way of the Poincaré-Hopf theorem, figuratively also referred to as the "hairy ball problem." Just as one can't comb a hairy ball flat without creating a cowlick, there will always be some microtubules that cannot lay flat against the membrane surface in a regular pattern. At certain locations the tubules will be oriented somewhat orthogonally to each other – in a very specific geometry. Since the microtubules in the case of the Munich researchers are in constant motion alongside each other due to the activity of the kinesin molecules, the faults also migrate. Amazingly, they do this in a very uniform and periodic manner, oscillating between two fixed orientations.
Spiked extensions
As long as the vesicle has a spherical shape, the faults have no influence on the external shape of the membrane. However, as soon as water is removed through osmosis, the vesicle starts to change in shape due to the movement within the membrane. As the vesicle loses ever more water, slack in the membrane forms into spiked extensions like those used by single cells for locomotion.
In this process, a fascinating variety of shapes and dynamics come to light. What seems random at first sight is, in fact, following the laws of physics. This is how the international scientists succeeded in deciphering a number of basic principles like the periodic behavior of the vesicles. These principles, in turn, serve as a basis for making predictions in other systems.
"With our synthetic biomolecular model we have created a novel option for developing minimal cell models," explains Bausch. "It is ideally suited to increasing the complexity in a modular fashion in order to reconstruct cellular processes like cell migration or cell division in a controlled manner. That the artificially created system can be comprehensively described from a physical perspective gives us hope that in the next steps we will also be able to uncover the basic principles behind the manifold cell deformations."
This research was supported in part by the German Excellence Initiative through the TUM Institute for Advanced Study and the Excellence Cluster Nanosystems Initiative Munich. The work of the Munich biophysicists was done in collaboration with colleagues from Brandeis University in Waltham , USA, the International School for Advanced Studies in Trieste, Italy and Syracuse University in New York, USA.
Publication:
Topology and dynamics of active nematic vesicles.
Felix C. Keber, Etienne Loiseau, Tim Sanchez, Stephen J. DeCamp, Luca Giomi, Mark J. Bowick, M. Cristina Marchetti, Zvonimir Dogic and Andreas R. Bausch. Science, 5 September 2014, DOI: 10.1126/science.1254784
Download High resolution pictures
Credit Video: Keber, Loiseau, and Bausch / TUM
Download Video (mov)
Download Video (flv)
Contact:
Prof. Andreas Bausch
Technische Universität München
Department of Physics
abausch@ph.tum.de
Tel: + 49 (89) 289-12408