First-ever “live” observation of formation and repair of myelin sheaths around nerve fibers
Watching myelin patterns form
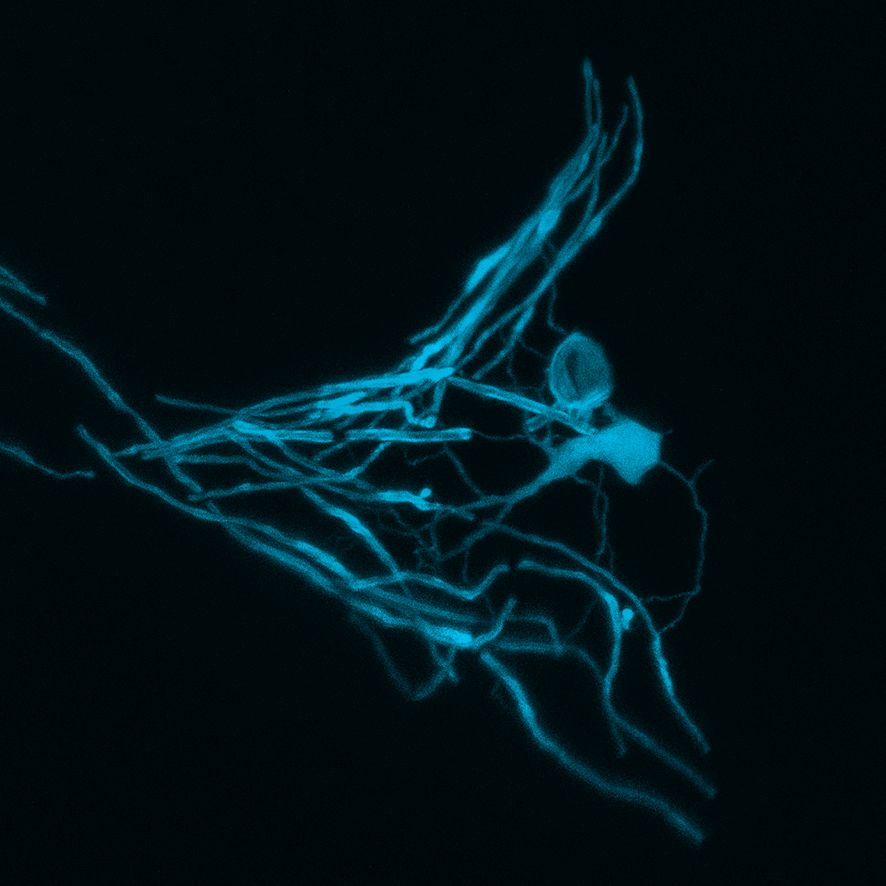
The myelin sheath surrounding the axons, or nerve fibers, can be compared to the insulation covering an electric wire. Without it, the rapid propagation of electric signals would not be possible. Damage to this insulating layer, for example from illnesses such as multiple sclerosis, may result in serious impairments.
Myelin segments determine the transmission speed
But myelin does not form a continuous coating along the axon. Instead, it is divided into segments. These can vary in length and are separated by gaps known as nodes of Ranvier. For the complex network of the central nervous system to function properly, the speed of the connections is not the only consideration. The crucial factor is the fine tuning: the signals have to reach the right place at exactly the right time. The transmission speed of information through an axon is partly determined by the number and length of the segments.
Patterns remain stable
The bodies of humans and animals have the ability – at least to some extent – to repair damaged myelin sheaths. Dr. Tim Czopka, a neuroscientist at TUM, has succeeded in making the first-ever "live" observations of this process. Czopka and his team used newly developed markers to visualize the formation myelin segments surrounding axons in the spinal cord of zebrafish.
They concluded: Characteristic patterns made up of myelin segments with different lengths along an axon are defined within a few days after myelin formation begins. Although the segments continue growing from that time onward – as the body of the zebrafish grows – the pattern remains preserved.
Following this observation, the researchers destroyed selected segments. “What happened next surprised us,” says Tim Czopka. “After the destruction of the segments, the myelin sheaths began to dynamically remodel. In the end, the damage was repaired and in most cases the original pattern was restored.” The regeneration followed a fixed sequence: First, the adjacent segments expanded to close the gap. A new segment then formed between them, and they contracted to their original size.
Axons influence the formation of segments
This raises an important question: Who controls the emergence and the restoration of the segment pattern? “Our observations suggest that it is not the oligodendrocytes – the cells that form myelin – that decide where it is formed, but rather the axons,” says Tim Czopka. “You could say that they know best which pattern is needed for the signals to be transmitted at optimal speed.”
The team is now studying how the segment patterns are affected by the targeted stimulation of nerve cell activity and by the neurotransmitters released as a result. “If we can understand the role of the axons in myelin generation and remodelling, it may yield new approaches to controlling it,” explains Czopka. “That would be relevant for the treatment of illnesses such as multiple sclerosis.”
Publication:
F. Auer, S. Vagionitis, T. Czopka, “Evidence for Myelin Sheath Remodeling in the CNS Revealed by In Vivo Imaging”, Current Biology (2018). DOI: 10.1016/j.cub.2018.01.017. (Open Access)
Contact:
Dr. Tim Czopka
Technical University of Munich
Institute of Neuronal Cell Biology
Tel: +49 89 4140-3377
tim.czopka@tum.de
Further Information:
Dr. Tim Czopka heads an Emmy Noether Junior Research Group at the Institute of Neuronal Cell Biology at TUM. The current research project is funded by the German Research Foundation (DFG). Dr. Tim Czopka has received funding from the European Research Council (ERC) under an ERC Starting Grant since 2016. He is a member of the Munich Cluster for Systems Neurology (SyNergy).