TUM researchers develop rapid test for severe infections
Risk of serious COVID-19 infection can now be predicted
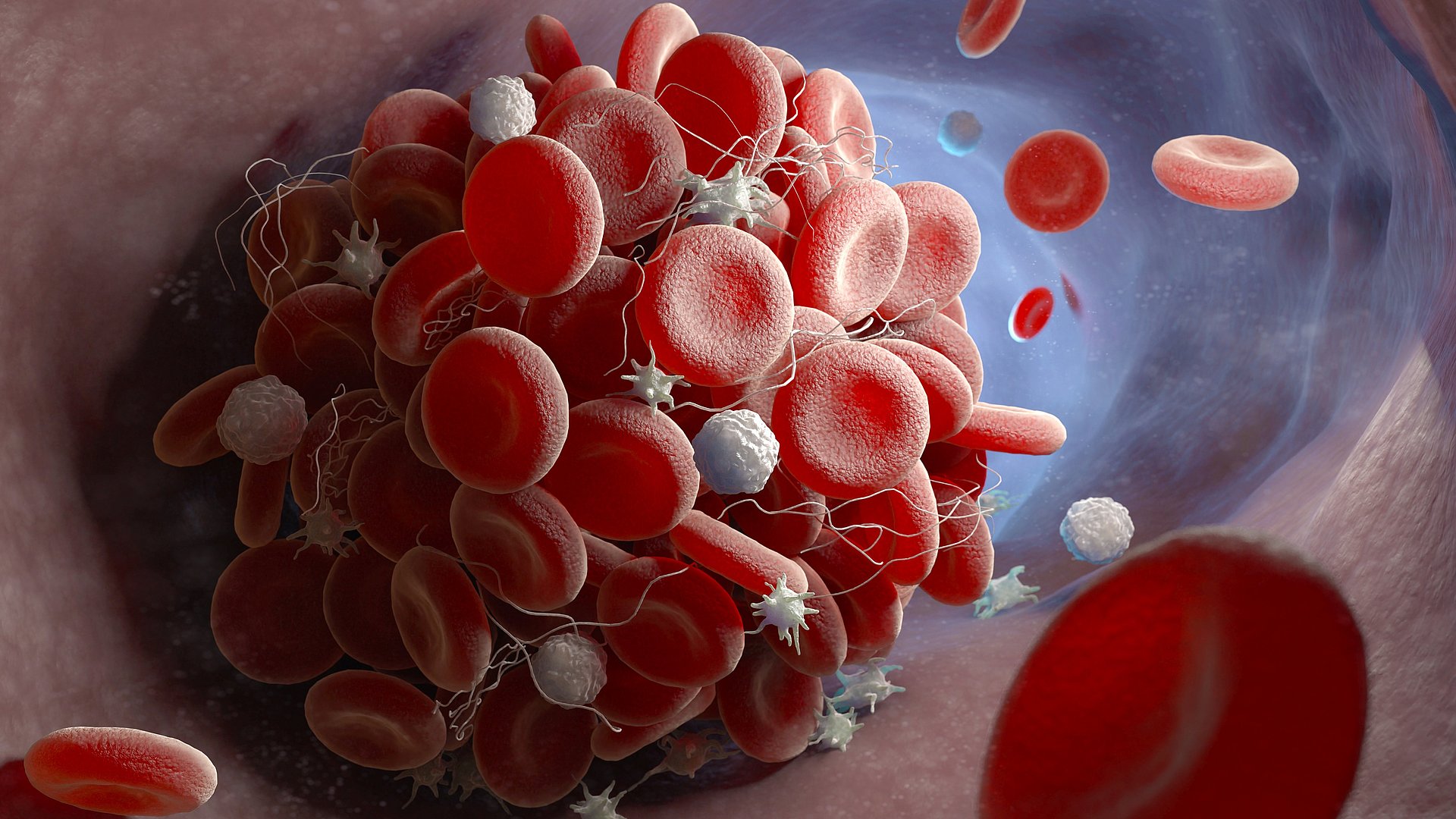
When infected with the Sars-CoV-2 virus, the human body produces a series of immune responses. One of them involves platelets, also known as thrombocytes, sticking to immune cells to form clumps, or aggregates, of cells in the bloodstream. In a study using image-based flow cytometry, a team of researchers working with Oliver Hayden, a professor of biomedical electronics, demonstrated a rapid rise in concentrations of platelet aggregates in patients admitted to intensive care with COVID-19 infections.
This enabled them to identify a biomarker for predicting the risk of severe illness in COVID-19 patients. The result was made possible by the optimal interdisciplinary conditions offered by the central TranslaTUM institute to TUM engineers for collaboration with medical researchers at Klinikum München rechts der Isar.
Proximity to patients and simple measurements
The analysis began with a blood sample taken from the test subjects. A few drops of blood are needed to count thousands of blood cells and their aggregates within seconds using image-based flow cytometry. The head of the study, Prof. Hayden, says: “Another big advantage of this method is that we neither have to treat nor mark the samples. Using standardized methods, we can investigate them directly without aggregation losses caused by high shearing forces. In the future, this cost-effective method could help quantify interactions between the coagulation system and the immune system.” The close proximity of patients to the lab when taking samples permits the blood to be tested immediately. This rules out effects from the aging of samples, which also causes aggregates to form.
Up to two-thirds of platelets in infected patients aggregated
The researchers investigated blood samples from 36 intensive care patients (age 32 to 83) admitted to the hospital with a SARS-CoV-2 infection classified as moderate to severe. The results showed that the number of aggregated thrombocytes in the blood samples of severely ill patients was significantly higher than in moderately ill patients and definitely in healthy blood donors.
With regard to the cell aggregates, the researchers found that the number of aggregates and their composition changed gradually in line with the severity of the COVID-19 infection and that these changes occurred at an early stage before complications appeared. The aggregates were typically made up of fewer than 10 thrombocytes. In extreme cases, it was observed that up to two thirds of all thrombocytes in a patient were aggregated.
Better patient management
A high concentration of cell aggregates was seen in all COVID-19 patients admitted to intensive care. This simple diagnostic method based on blood cell aggregates has the potential to identify high-risk patients at an early stage and improve their care.
The interdisciplinary team of engineers and medical researchers now plan to transfer what they have learned to other diseases. They believe that the method described here could also function with cardiovascular disease or cancers.

Klenk, C., Erber, J., Fresacher, D., Röhrl, S., Lengl, M., Heim, D., Irl, H., Schlegel, M., Haller, B., Lahmer, T., Diepold, K., Rasch, S. and Hayden, O. Platelet aggregates detected using quantitative phase imaging associate with COVID-19 severity. Commun Med 3, 161 (2023). doi.org/10.1038/s43856-023-00395-6.
- Professor Hayden conducts research aimed at the development of innovative ways of using in-vitro techniques for diagnostic and biomedical purposes. Since 2017 he has held the Heinz Nixdorf Chair for Biomedical Electronics.
- The Heinz Nixdorf Chair of Biomedical Electronics at the TUM School for Computation, Information and Technology develops new diagnostic methods at the Central Institute for Translational Cancer Research at the Technical University of Munich (TUM) (TranslaTUM).
- The goal of the CellFACE project (Chair of Data Processing & Heinz Nixdorf Chair for Biomedical Electronics) is to validate imaging flow cytometry using light phases as a label-free platform technology for acute care. To ensure the success of the project, back-to-back teams between Munich and Singapore were formed.
Technical University of Munich
Corporate Communications Center
- Julia Rinner
- julia.rinner@tum.de
- presse@tum.de
- Teamwebsite
Contacts to this article:
Prof. Oliver Hayden
Technical University of Munich
Heinz Nixdorf Chair of Biomedical Electronics
Email: oliver.hayden@tum.de
Tel: +49 (89) 4140 - 9031