New technique enables detailed insights into mitochondria
Neuroimaging: Live from inside the cell
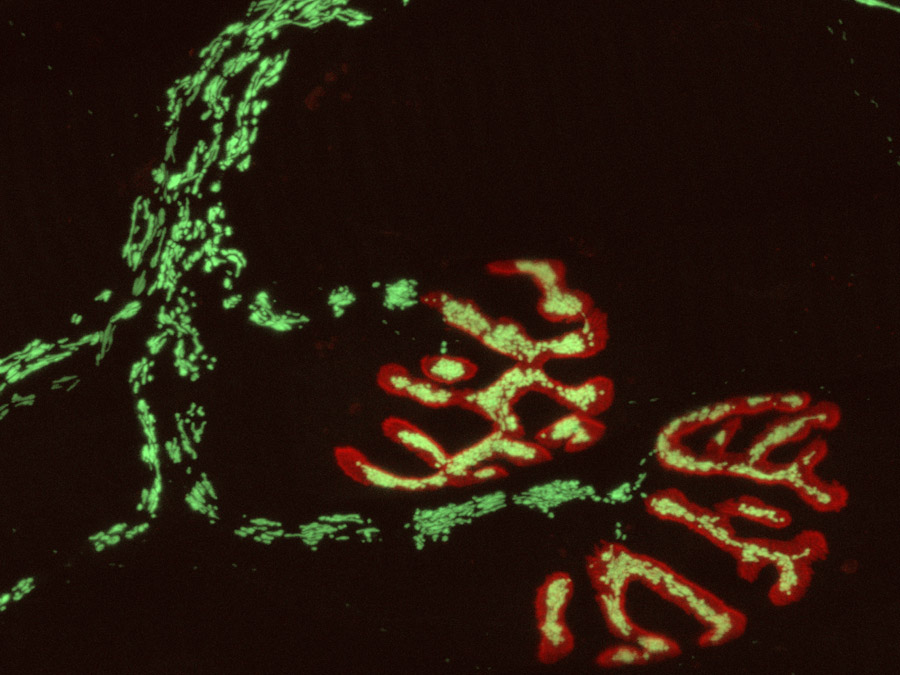
Reactive oxygen species are important intracellular signaling molecules, but their mode of action is complex: In low concentrations they regulate key aspects of cellular function and behavior, while at high concentrations they can cause “oxidative stress”, which damages organelles, membranes and DNA. To analyze how redox signaling unfolds in single cells and organelles in real-time, an innovative optical microscopy technique has been developed jointly by the teams of LMU Professor Martin Kerschensteiner and TUM Professor Thomas Misgeld, both investigators of the Munich Cluster for Systems Neurology (SyNergy).
“Our new optical approach allows us to visualize the redox state of important cellular organelles, mitochondria, in real time in living tissue” Kerschensteiner says. Mitochondria are the cell’s power plants, which convert nutrients into usable energy. In earlier studies, Kerschensteiner and Misgeld had obtained evidence that oxidative damage of mitochondria might contribute to the destruction of axons in inflammatory diseases such as multiple sclerosis.
The new method allows them to record the oxidation states of individual mitochondria with high spatial and temporal resolution. Kerschensteiner explains the motivation behind the development of the technique: “Redox signals have important physiological functions, but can also cause damage, for example when present in high concentrations around immune cells.”
First surprises
Kerschensteiner and Misgeld used redox-sensitive variants of the Green Fluorescent Protein (GFP) as visualization tools. “By combining these with other biosensors and vital dyes, we were able to establish an approach that permits us to simultaneously monitor redox signals together with mitochondrial calcium currents, as well as changes in the electrical potential and the proton (pH) gradient across the mitochondrial membrane,” says Thomas Misgeld.
The researchers have applied the technique to two experimental models, and have arrived at some unexpected insights. On the one hand, they have been able, for the first time, to study redox signal induction in response to neural damage – in this case, spinal cord injury – in the mammalian nervous system. The observations revealed that severance of an axon results in a wave of oxidation of the mitochondria, which begins at the site of damage and is propagated along the fiber. Furthermore, an influx of calcium at the site of axonal resection was shown to be essential for the ensuing functional damage to mitochondria.
Perhaps the most surprising outcome of the new study was that the study's first author, graduate student Michael Breckwoldt, was able to image, also for the first time, spontaneous contractions of mitochondria that are accompanied by a rapid shift in the redox state of the organelle. As Misgeld explains, “This appears to be a fail-safe system that is activated in response to stress and temporarily attenuates mitochondrial activity. Under pathological conditions, the contractions are more prolonged and may become irreversible, and this can ultimately result in irreparable damage to the nerve process.”
Publication
Michael O. Breckwoldt, Franz Pfister, Peter M. Bradley, Petar Marinković, Philip R. Williams, Monika S. Brill, Barbara Plomer, Anja Schmalz, Daret K. St. Clair, Ronald Naumann, Oliver Griesbeck, Markus Schwarzländer, Leanne Godinho, Florence M. Bareyre, Tobias P. Dick, Martin Kerschensteiner and Thomas Misgeld, Multi-parametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology, Nature Medicine (2014).
DOI: 10.1038/nm.3520
Contact:
Prof. Dr. Martin Kerschensteiner
Institute of Clinical Neuroimmunology, Ludwig-Maximilians Universität München (LMU)
Phone: +49 89 2180 78282
Martin.Kerschensteiner@med.uni-muenchen.de
AG Kerschensteiner
Prof. Dr. Thomas Misgeld
Institute of Neuroscience, Technische Universität München (TUM)
Phone: +49 89 4140 3512
thomas.misgeld@lrz.tum.de
Misgeld-Lab
Technical University of Munich
Corporate Communications Center
- Nicola Holzapfel (LMU) / Dr. Vera Siegler
- siegler@zv.tum.de
- presse@tum.de
- Teamwebsite