Interaction between gut bacteria and mucus layer cells
Cause of inflammatory bowel disease discovered
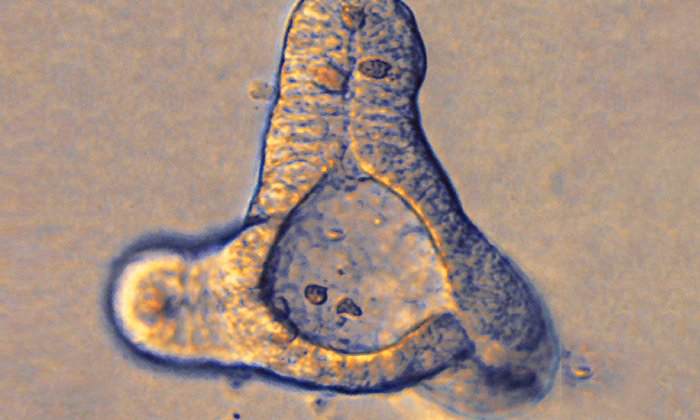
The billions of bacteria living in the human gut – known collectively as the microbiome – are of enormous importance. They help with digestion, among other functions. Consequently, the immune system in the gut must be extremely well regulated: It should fight only harmful pathogens without attacking useful microorganisms. However, this fine balance can be disrupted by various factors.
A defect in the gene XIAP, which causes the rare disease XLP2, results in chronic inflammation of the bowels in 30 percent of all cases, among other symptoms. Babies with this genetic defect often display serious symptoms such as diarrhea, abdominal pain, weakness and weight loss soon after birth. Until now, scientists have been unable to understand the underlying mechanism or discover effective treatments – apart from stem cell transplants, which involve a high risk of mortality.
Overreaction of the innate immune system
Working with organoids – intestinal cells in a Petri dish – and animal experiments, a team headed by Dr. Monica Yabal, Adam Wahida and Madeleine Müller of the Institute for Molecular Immunology and the Clinic of Hematology and Oncology of TUM’s university hospital, Klinikum rechts der Isar, has now identified the mechanism behind the inflammation response and learned how it becomes chronic. “The innate immune system overreacts to microbes in the gut,” says Yabal. The immune system in healthy people eliminates bacteria that cause illness and then returns to its resting state. But in some XLP2 patients, a fatal chain reaction begins:
Every person has toll-like receptors (TLRs) that use unique structures such as molecules in the cell wall to identify harmful microbes. When a TLR binds a molecule, the signaling substance TNF and its TNFR1 and TNFR2 receptors activate the immune system to eliminate the pathogen. However, this does not work properly in XLP2 patients. Instead, the binding of TNF to the TNFR1 receptor on cells known as Paneth cells causes these cells to die, resulting in a vicious circle. That is because the Paneth cells in the gut mucus layer produce antimicrobial substances and thus ensure a bacterial balance in the intestines. The loss of those cells changes the composition of the microbiome. Beneficial bacteria such as clostridia are attacked and can no longer perform their regulatory role. This again activates the immune system.
New drugs could stop the inflammation response
“We believe that this principle might also be applicable to other inflammatory bowel diseases and not only in XLP2 patients,” says Prof. Percy Knolle, the Director of the Institute for Molecular Immunology at TUM. Malfunctioning Paneth cells have also been observed in many patients with inflammatory bowel diseases with various causes.
These insights might open up important avenues for the development of new drugs. Patients with chronic bowel inflammation have been treated for many years with drugs that inhibit the TNF receptors. However, these molecules are not very specific and deactivate both TNFR1 and TNFR2. “Our experiments show that it would be better if we had a selective inhibitor for the TNFR1 receptor,” says Yabal. It also remains unclear why some people respond very well to this treatment while others show no response at all. Consequently, the team would now like to turn its attention to the adaptive immune system, which learns throughout an individual’s lifetime through contact with pathogens and forms special antigens, and also study its special role in the gut.
Adam Wahida, Madeleine Müller, Andreas Hiergeist, Bastian Popper, Katja Steiger, Caterina Branca, Markus Tschurtschenthaler, Thomas Engleitner, Sainitin Donakonda, Jordy De Coninck, Rupert Öllinger, Marie K. Pfautsch, Nicole Müller, Miguel Silva, Sinem Usluer, Erik Thiele Oberg, Jan P. Böttcher, Nicole Pfarr, Martina Anton, Julia B. Slotta-Huspenina, Andreas G. Nerlich, Tobias Madl, Marijana Basic, André Bleich, Geert Berx, Jürgen Ruland, Percy A. Knolle, Roland Rad, Timon E. Adolph, Peter Vandenabeele, Hirokazu Kanegane, André Gessner, Philipp J. Jost, Monica Yabal. XIAP restrains TNF-driven intestinal inflammation and dysbiosis by promoting innate immune responses of Paneth and dendritic cells (2021) Science Immunology, Vol 6, Issue 65. DOI: 10.1126/sciimmunol.abf7235.
The study was funded by the European Research Council (ERC), the German Research Foundation (DFG), the Austrian Research Promotion Agency (FFG) and the German Center for Infection Research (FFG).
Contacts to this article:
Dr. Monica Yabal
Institute of Molecular Immunology
Klinikum rechts der Isar / Technical University of Munich (TUM)
Tel: +49 89 4140 9748
monica.yabal@tum.de