A simple catalyst helps to construct complex biological scaffolds
From the scent of geranium to cough medicine
With great elegance nature builds up complex structures from simple building blocks. A central class of compounds are terpenes. More than 8000 terpenes and about 30,000 of the related terpenoids are currently known. They are the key substances for many biological and pharmaceutical functions.
Eucalyptol, or 1.8-cineole, for example is contained in many medicines for cough. It is an expectorant and works bactericidal. Chemically it consists of a ring of six carbon atoms which is additionally bridged. Starting out from geraniol, the main constitutend of the scent of geranium, this double ring is formed by a so-called tail-head-cyclization.
The biggest challenge of an artificial production is that as a first step a high-energy intermediate state is formed, in which the molecule has a positive charge. Without a catalyst, the molecule could further react in different directions. The desired product would be one of many and the yield only low.
“Our catalyst stabilizes the transition state and directs the reaction in the right direction,” says Konrad Tiefenbacher, Professor of Organic Chemistry at the Technische Universität München. “In solution these reactions were previously not feasible.”
Self-assembled catalyst
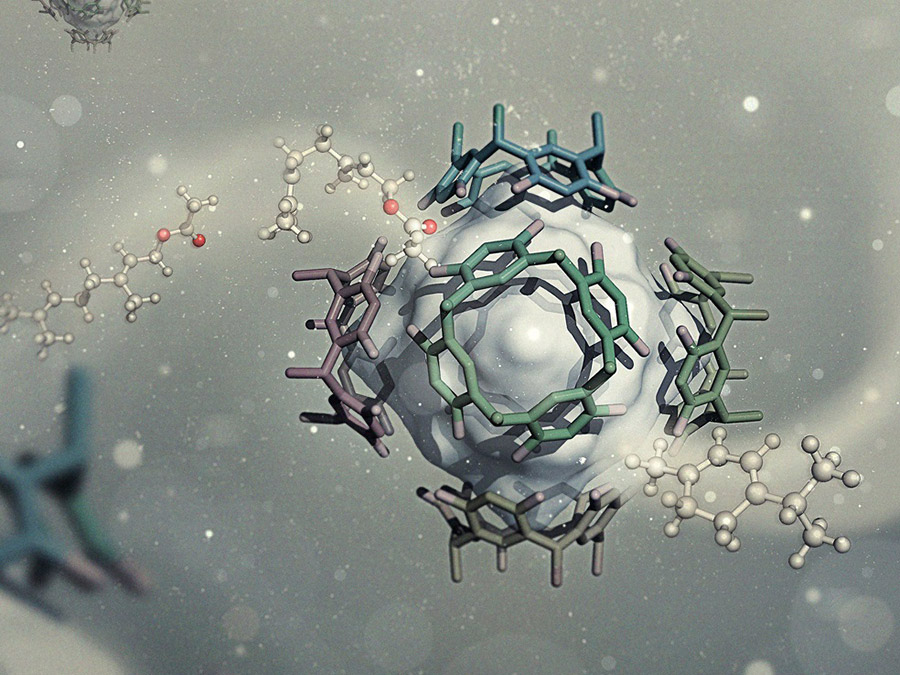
Also the catalyst of the reaction is special: four resorcinol molecules are linked to form a large ring consisting of 16 carbon atoms. Six of these molecules self-assemble in solution to a large, octahedron-like cage. In its interior the cyclization reaction proceeds.
The electron-rich aromatic ring systems of the resorcinol-blocks appear to stabilize the positive charge of the intermediate state. Similar to the reaction pocket of the cyclase enzyme of the eucalyptus tree, the catalyst thus prevents undesirable side reactions.
Using other parent compounds than geraniol a variety of other products could be feasible. “Eucalyptol is only a first step,” says Konrad Tiefenbacher. “Our ultimate goal is the production of compounds with much higher complexity, such as taxol, which is used in the fight against cancer.”
Publication:
Terpene cyclization catalysed inside a self-assembled cavity
Q. Zhang and K. Tiefenbacher
Nature Chemistry, Advanced Online Publication, 16. Februar 2015 - DOI: 10.1038/nchem.2181
Contact:
Prof. Dr. Konrad Tiefenbacher
Technische Universität München
Lichtenbergstr. 4, 85747 Garching, Germany
Tel.: +49 89 289 13332 – E-mail – Internet