A Calcium channel is involved in the development of pancreatitis
A disease trigger for pancreatitis has been identified
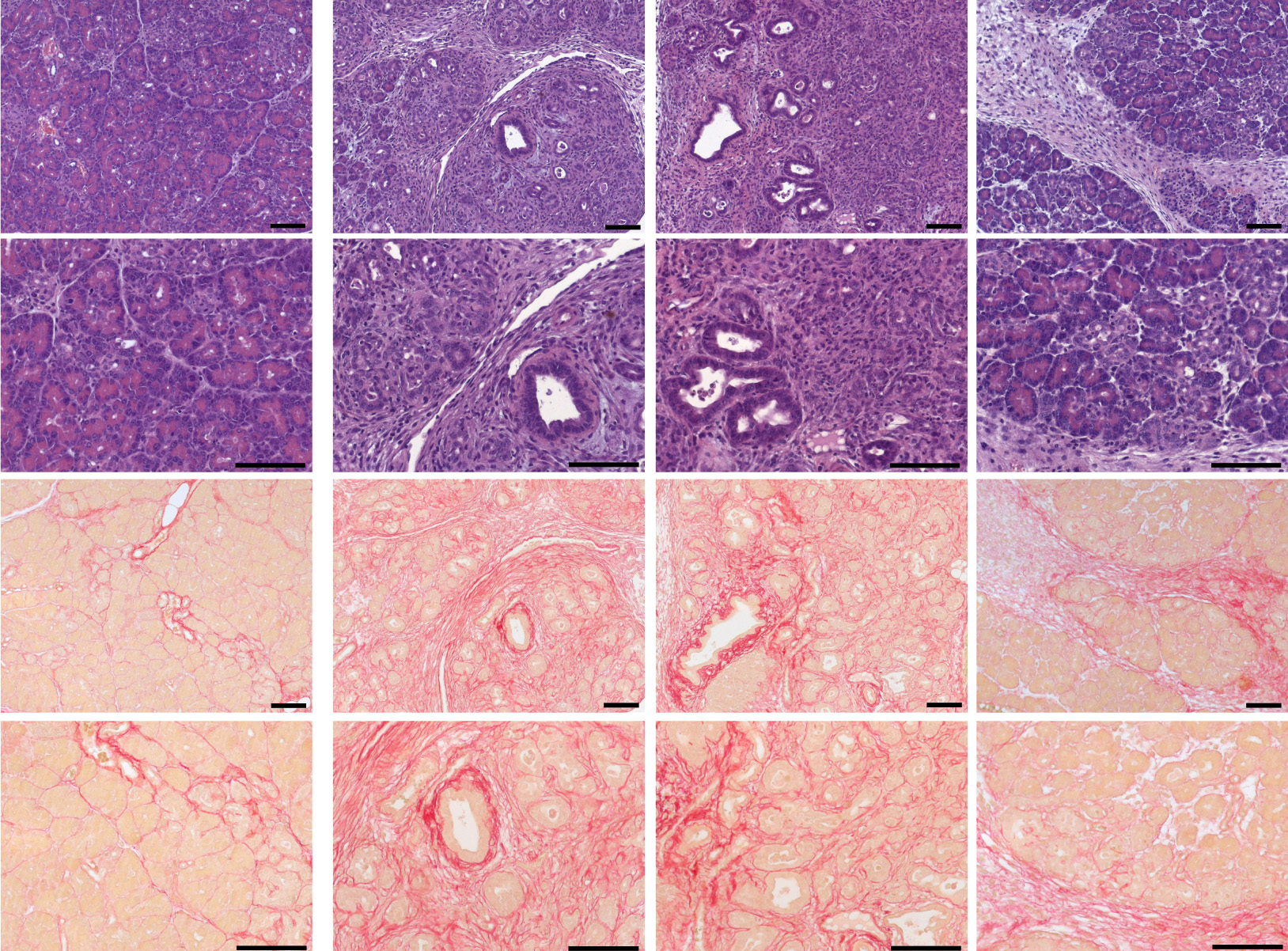
Patients suffering from chronic pancreatitis experience an either recurring or permanent inflammation of their pancreas. “In many cases, people develop this disease because they are drinking too much alcohol or they are smoking too much. Certain medication or high levels of lipids or calcium in a patient’s blood can be another cause of pancreatitis,” explained Heiko Witt, one of the two heads of the study and Professor for Pediatric Nutritional Medicine at the Else Kröner-Fresenius-Zentrum (EKFZ) at TUM.
The main focus of previous research was on the so-called acinus cells of the pancreas; these are responsible for creating digestive enzymes. Many patients suffering from genetically caused pancreatitis show mutations in digestive enzymes or in molecules inhibiting the enzymes’ effectiveness.
Calcium channel disorder leads to pancreatitis
In the course of the current study, that was performed with European and Japanese patients suffering from pancreatitis not associated with alcohol consumption, the researchers discovered that genetic defects which were heavily limiting the functionality of calcium channel TRPV6 caused early-onset chronic pancreatitis.
“A substantial TRPV6 defect is a globally occurring and serious risk factor for developing chronic pancreatitis,“ said Professor Witt and added: “By identifying alterations of the calcium channel, we are now also considering duct cells as part of the concept covering the origins of this disease.” Duct cells serve as coating in the channels that transport digestive enzymes from the point of origin into the intestines.
Using a mouse model, the scientists were able to show that the absence of the corresponding gene would, in most cases, lead to inflammation and the occurrence of fibrotic changes in the pancreas, which is typical for chronic inflammation.
This new discovery offers opportunities for pharmacologic therapy approaches
The discovery that a calcium channel malfunction can contribute to the development of pancreatitis offers a new course of action for therapeutic intervention. Furthermore, the research findings will become part of the standard diagnostics for genetically caused pancreatitis.
These insights also pave the way for a new area of research covering the investigation of causes of pancreatitis – no longer focused on acinus cells and digestive enzymes but rather on duct cells and channels as well as calcium metabolism.
The identification of mutations in a calcium channel as a (contributing) cause of disease leads to new factors – other calcium channels and proteins that are relevant for calcium metabolism – moving into the scientific focus. “Currently we are investigating these genes for genetic alterations and we have a European patient collective comprising 1100 people with pancreatitis to gather our data,” reported Professor Witt.
He further explained: “Deciphering the genetic backgrounds of pancreatitis will substantially affect our understanding of these types of genetically caused pancreatitis, as well as our understanding of alcohol-related pancreatitis. It will enable new research approaches which may lead to new treatment possibilities in the future.”
Masamune A., Kotani H., Sörgel F.L., Chen J.M., Hamada S., Sakaguchi R., Masson E., Nakano E., Kakuta Y., Niihori T., Funayama R., Shirota M., Hirano T., Kawamoto T., Hosokoshi A., Kume K., Unger L., Ewers M., Laumen H., Bugert P., Mori M.X., Tsvilovskyy V., Weißgerber P., Kriebs U., Fecher-Trost C., Freichel M., Diakopoulos K.N., Berninger A., Lesina M., Ishii K., Itoi T., Ikeura T., Okazaki K., Kaune T., Rosendahl J., Nagasaki M., Uezono Y., Algül H., Nakayama K., Matsubara Y., Aoki Y., Férec C., Mori Y., Witt H., Shimosegawa T.: Variants That Affect Function of Calcium Channel TRPV6 Are Associated With Early-Onset Chronic Pancreatitis. Gastroenterology. 2020 Jan 10. pii: S0016-5085(20)30017-2. DOI: 10.1053/j.gastro.2020.01.005.
This paper was supported by the Else Kröner-Fresenius-Zentrum (EKFZ). Professor Witt is a member of the Else Kröner-Fresenius-Zentrum for Nutritional Medicine (EKFZ). EKFZ was established at TUM in the year 2005: Their innovative approach combines traditional nutritional science with medical research on a wide spectrum – an entirely new approach in the European research landscape. (https://www.tum.de/nc/die-tum/aktuelles/pressemitteilungen/details/34829/)
Several institutions participated in creating this study. In Germany, these are the participants: Professorship for Pediatric Nutritional Medicine, Else Kröner-Fresenius-Zentrum for Nutritional Medicine (EKFZ), TUM, Prof. Dr. Heiko Witt, the Comprehensive Cancer Center at the University Hospital Klinikum rechts der Isar (MRI) of TUM, Prof. Dr. Hana Algül, the Pharmacological Institute at the University of Heidelberg & DZHK (German Center for Cardiovascular Research), the department of Experimental and Clinical Pharmacology and Toxicology at Saarland University, as well as the Department of Internal Medicine at Martin Luther University Halle-Wittenberg. In Japan, the following institutions were involved: the Division of Gastroenterology & Division of Medical Genetics & Division of Cell Proliferation at Tohoku University Graduate School of Medicine, Sendai, as well as the Department of Synthetic Chemistry and Biological Chemistry, Kyoto. In France, the paper was supported by the INSERM Organization at the University of Western Brittany at Brest.
Technical University of Munich
Corporate Communications Center
- Dr. Katharina Baumeister
- katharina.baumeister@tum.de
- 08161-71-5403
- presse@tum.de
- Teamwebsite
Contacts to this article:
Prof. Dr. Heiko Witt
Technical University of Munich
Professor of Paediatric Nutritional Medicine
Else Kröner-Fresenius Zentrum (EKFZ)
phone: +49.8161.71.2466
Heiko.Witt@tum.de

