New method for labeling T cells in immunotherapy
Tracking immune cells inside the body
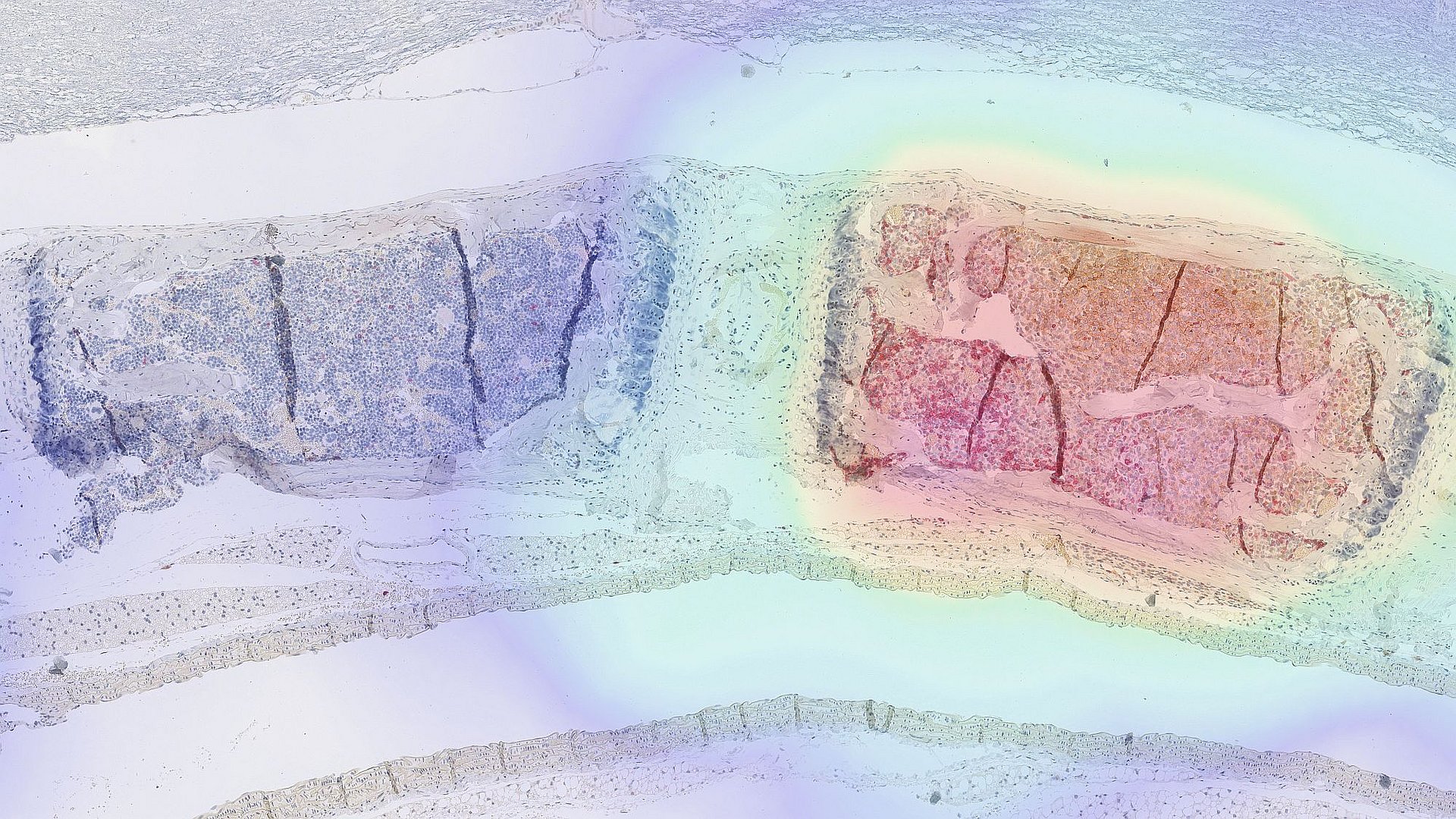
When standard treatments for diseases like cancer fail, custom-tailored cell therapies are increasingly becoming a viable option. A prominent example is CAR T-cell therapy. In this approach, immune cells are taken from the patient and genetically engineered in the lab to carry a receptor that recognizes structures specific to the surface of cancer cells. These modified immune cells then multiply in the body and initiate an immune response against the tumor.
Physicians could greatly benefit from knowing exactly how these modified immune cells behave in the body: Do they migrate to where they are needed? Do they replicate sufficiently? Do they behave unpredictably and, in the worst-case scenario, attack healthy tissue? Currently, there are no clinically applicable methods to answer these critical questions.
An artificial receptor and a custom-designed marker
An interdisciplinary team at TUM and the TUM University Hospital has now proposed a solution. In simplified terms, a second artificial receptor is inserted into the modified immune cells. These cells can then be visualized using PET imaging and a specially developed, non-toxic radioactive contrast agent. When this so-called radioligand is injected into the body, it binds exclusively to the modified cells and their descendants, making them visible.
The technique relies on artificial proteins with specific binding properties, known as anticalins. These have been developed since the 1990s by Arne Skerra, Professor of Biological Chemistry at TUM and a pioneer in protein engineering. His work led to the creation of an anticalin that binds the ligand DTPA and has now been adapted as part of a cell surface receptor. A team led by Wolfgang Weber, Professor of Nuclear Medicine at TUM University Hospital, used this concept to engineer an artificial gene that causes cells to express the anticalin receptor "DTPA-R" on their surface. The project was spearheaded by Volker Morath and Katja Fritschle from the Department of Nuclear Medicine, who, along with their team, also developed the matching radioligand for DTPA-R: 18F-DTPA. The method was tested on CAR T cells in collaboration with immunotherapy expert Dirk Busch, Professor of Medical Microbiology, Immunology and Hygiene at TUM.
Expectations met
In experiments with mice, the researchers were able to demonstrate that the modified cells indeed migrated to the affected diseased tissue and proliferated there. They also showed that the radioligand is rapidly excreted via the kidneys, binds exclusively to cells with the artificial receptor, and does not interfere with other processes in the body. Moreover, the study showed that this approach can also be used to monitor gene therapies in which viruses serve as tools to alter genetic information within cells.
"An important tool"
“For several years now, it has been clear that new medical applications like immunotherapies and gene therapies hold tremendous potential,” says Prof. Wolfgang Weber, who led the study. “We believe that we have created a valuable tool that can make such therapies safer by providing better insight into what happens inside the body.” The technique is still in its early stages. Before it can be used in human patients, its safety and efficacy must be verified in clinical trials. Further development toward clinical trial use and commercialization is currently ongoing.
Still, the researchers believe the method can already yield valuable insights for basic research. It is also intended to support animal welfare: If laboratory animals can be continuously monitored during experiments, their numbers could be significantly reduced in the development of new cell and gene therapies.
Volker Morath, Katja Fritschle et al. „PET-based tracking of CAR T cells and viral gene transfer using a cell surface reporter that binds to lanthanide complexes”. Nature Biomedical Engineering (2025). DOI: 10.1038/s41551-025-01415-7.
- The ongoing development of the approach is supported by the GO-Bio initial program of the German Federal Ministry of Research, Technology and Space and the UK-based National Centre for the 3Rs.
-
Also contributing significantly to the study was PD Dr. Katja Steiger from the Institute of Pathology at TUM.
-
Research into methods for tracking immune cells using imaging techniques is underway in many places. At TUM, for example, the start-up ImuVeo – which also has its roots in the Department of Nuclear Medicine and the Department of Medicine III at TUM University Hospital – has developed a technique that enables immune cell tracking.
Technical University of Munich
Corporate Communications Center
- Paul Hellmich
- paul.hellmich@tum.de
- presse@tum.de
- Teamwebsite
Contacts to this article:
Prof. Wolfgang Weber
Technical University of Munich
TUM University Hospital
Department of Nuclear Medicine
Tel. +49 (89) 4140 2972
nukmed.sekretariat@mri.tum.de