Bilayer structural makeup of biomembranes
Pathway for membrane building blocks
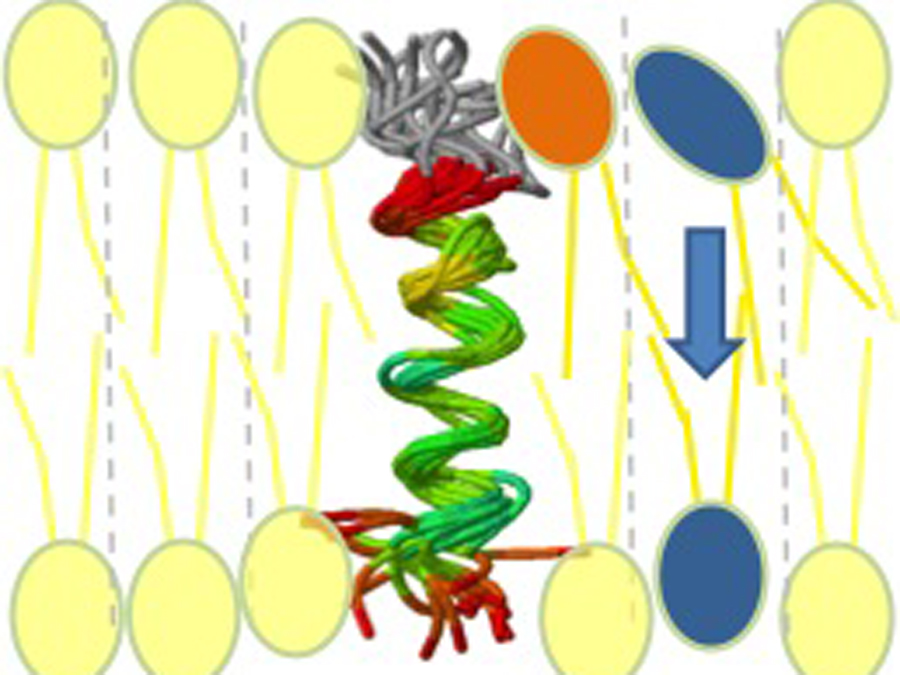
The lipid molecules of membranes, also known as phospholipids, are composed of two elements: A hydrophilic head and two long-chain fatty acids. The molecules form a bilayer in the membrane, with all of the heads pointing outwards and the fatty acid chains hanging in a zip-like interlay position.
Biomembranes are constantly reorganized or renewed, for example whenever cells divide. The cell is constantly creating new phospholipids that have to align themselves – which they do in both layers of the biomembrane. However, cells only produce phospholipids on one side of the biomembrane. From there, they need to be transported to the other half of the bilayer.
A helping hand through the membrane
The problem is that the hydrophilic and lipophilic parts of the molecule repel each other. “The molecules can anchor themselves in one of the two membrane layers with their lipophilic tail,” explains Prof. Dieter Langosch of the TUM Chair of Biopolymer Chemistry. “Translocation to the second layer is not possible because the hydrophilic heads cannot pass through the lipophilic fatty acid chains.”
The key to establishing order in the membranes lies in enzymes that transport the molecules to their correct location in the “second layer”. Scientists have been searching for such enzymes – known as flippases – for many years. But now Prof. Langosch and his team have made a breakthrough. They experimented with synthetic peptides, which transport phospholipids through the membrane.
A clever trick
In this process, the researchers came across an indirect transport mechanism. The peptides span both layers of the membrane – and are able to bind to individual phospholipids. Prof. Langosch explains: “When the peptides bind the molecules, the surrounding membrane is briefly destabilized. The new phospholipids use this opportunity to slip through the barrier of the first lipid layer and flip to the second layer of the membrane.”
The researchers now have a clear idea of how flippases work. “Our peptides stretch through the membrane like a corkscrew. If this “alpha-helix” has dynamic elements, it can bind to phospholipids,” says Prof. Langosch. “This model will help us to detect the flippases.”
Publication:
Structural Properties of Model Phosphatidylcholine Flippases: Marcella Langer, Rashmi Sah, Anika Veser, Markus Gütlich, Dieter Langosch; CELL, Chemistry & Biology; Volume: 20; Issue: 1; 2012, doi:10.1016/j.chembiol.2012.11.006.
Contact:
Prof. Dr. Dieter Langosch
Technische Universität München
Lehrstuhl für Chemie der Polymere
T: +49.861.71.3500
E: langosch@tum.de
W: www.wzw.tum.de/biopolymere/