Computer model shows neuronal activation patterns in the inner ear
Research towards improved cochlear implants

People with normal hearing perceive sound by means of hair cells located in the cochlea, the fluid-filled hollow part of the inner ear. These hair cells convert sonic vibrations into auditory nerve impulses which are then passed on to the brain where they cause hearing sensations.
For several decades it has now been possible to restore hearing in people with deafness or severe hearing loss at an amazingly high level with the help of cochlear implants. The implants use an external microphone to capture sound information from the air and transfer it to implanted electrodes. These electrodes directly stimulate the auditory nerve fibers in the inner ear with electric impulses, so that the patient can perceive sound once again.
Imprecise auditory perception
The special structure of the inner ear makes hair cells at different locations in the cochlea sensitive to different pitches. We perceive the impulses transmitted by the docked nerves as tones with the respective corresponding pitch. The electrodes of a cochlear implant are also positioned at various points along the cochlea. When sound of a particular pitch reaches the implant's microphone, a specific electrode emits electrical signals.
However, one electrode not only activates the nerve fibers in its immediate vicinity, but as current spreads widely in the salt-water filled inner ear, it also activates nerve fibers at more remote areas of the cochlea. As a result, cochlear-implant users cannot differentiate between impulses corresponding to electrode contacts located too close to each other. This limits the number of useful electrode contacts in actual implants.
Computer model shows signal spread
Understanding the best possible positioning of the electrode contacts requires knowledge of how the signals of the individual electrodes activate the nerve fibers. Researchers in the working group of Werner Hemmert, Professor for Bio-Inspired Information Processing at the Technical University of Munich (TUM), have come a major step closer to achieving this goal. They have developed a complex computer model that precisely calculates the spread of the electrical signals in the inner ear.
First, working together with colleagues at the university hospital TUM Klinikum rechts der Isar, the team used high-resolution computed tomography to create a three-dimensional representation of the bone containing the cochlea. “The representation also showed the fine pores through which the auditory nerve fiber bundles pass,” explains Siwei Bai, post-doctoral researcher in Hemmert's research group and first author of the study. Using an algorithm developed by the group, the three-dimensional micro-structures of these pores could be used to reconstruct the paths of the individual nerve fibers, running from the cochlea through the bone all the way to the brain.
More complex than expected
“We were surprised at how unevenly the nerve fibers react to the implant's electrical signals: Some are very sensitive and are easily activated by almost all electrodes. Others are less sensitive and are mainly stimulated by the electrodes closest to them,” says Hemmert. “This is a result of fine anatomical differences and the exact trajectories of the auditory nerve fibers.” Thus, it cannot be generally assumed that a given electrode will have a stronger effect on nerve fibers in its proximity than on more distant nerve fibers. Up to now, researchers had constructed radial-symmetric models that predicted a uniform decay of the sensitivity of the auditory nerve fibers with distance to the electrode. The new findings however show how important it is to begin with a precise representation of the irregular bone and auditory nerve fibers.
Optimizing implants – Improving quality of life
In a next step, the researchers plan to incorporate the exact structure of the individual nerve fibers in their model. Then, they will be able to determine exactly where the electric pulses stimulate the nerve fibers and how the pulses spread along the fibers. “All these results will then be used in the development of new implants which will improve the quality of the stimulation, improving language understanding and ultimately also the patient's quality of life,” Hemmert concludes.
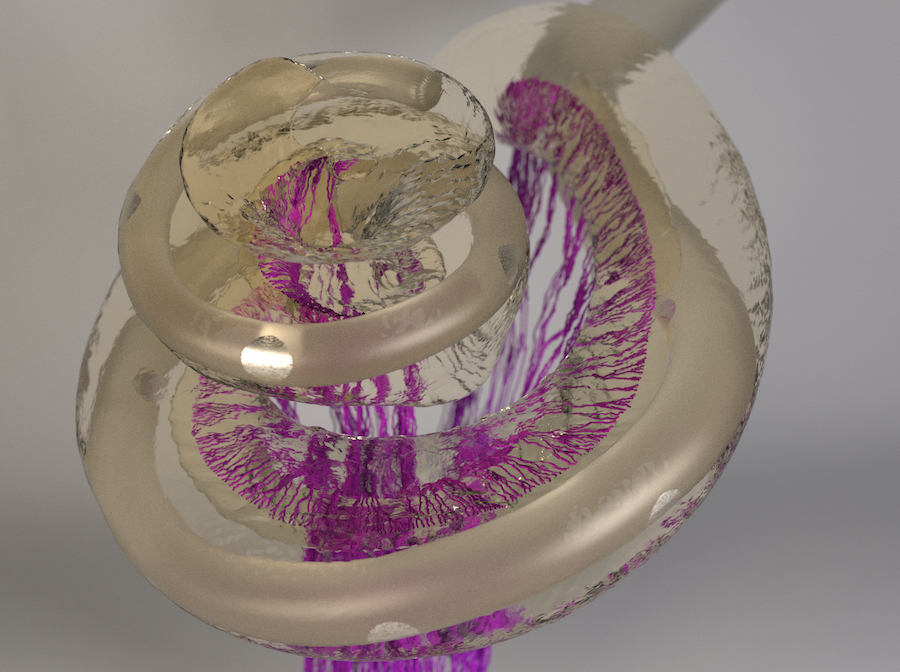
Electrical Stimulation in the Human Cochlea: A Computational Study Based on High-Resolution Micro-CT Scans
Siwei Bai, Jörg Encke, Miguel Obando-Leitón, Robin Weiß, Friederike Schäfer, Jakob Eberharter, Frank Böhnke and Werner Hemmert
Frontiers in Neuroscience, 05. Dezember 2019, DOI: 10.3389/fnins.2019.01312
Comparison of Multi-Compartment Cable Models of Human Auditory Nerve Fibers
Richard Bachmaier, Jörg Encke, Miguel Obando-Leitón, Werner Hemmert and Siwei Bai
Frontiers in Neuroscience, 05. November 2019 DOI: 10.3389/fnins.2019.01173
Prof. Werner Hemmert and Dr. Siwei Bai work at the Munich School of BioEngineering. This interdisciplinary TUM research center is Europe's most multi-disciplinary university institution focused on the interface between medicine, engineering and natural sciences.
Werner Hemmert is also member of the Bernstein Center for Computational Neuroscience (BCCN), the German Society for Audiology (DGA) and the Association for Research in Otolaryngology (ARO).
This project and the authors were supported by the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 702030, and the German Research Foundation (DFG) under the D-A-CH programme (HE 6713/2-1).
Video on cochlea implant research of Prof. Hemmert's group (YouTube)
Technical University of Munich
Corporate Communications Center
- Paul Piwnicki
- paul.piwnicki@tum.de
- +49 89 289 10808
- presse@tum.de
- Teamwebsite
Contacts to this article:
Prof. Dr. Werner Hemmert
Technical University of Munich
Bio-Inspired Information Processing
Munich School of BioEngineering
Phone:+49 89 289 10853
E-Mail: werner.hemmert(at)tum.de
Dr. Siwei Bai
Technical University of Munich
Bio-Inspired Information Processing
Munich School of BioEngineering
Phone: +49 89 289 10842
E-Mail: siwei.bai(at)tum.de



