Mutations in plasma cells play a key role in light chain amyloidosis
Fatal overproduction of antibodies

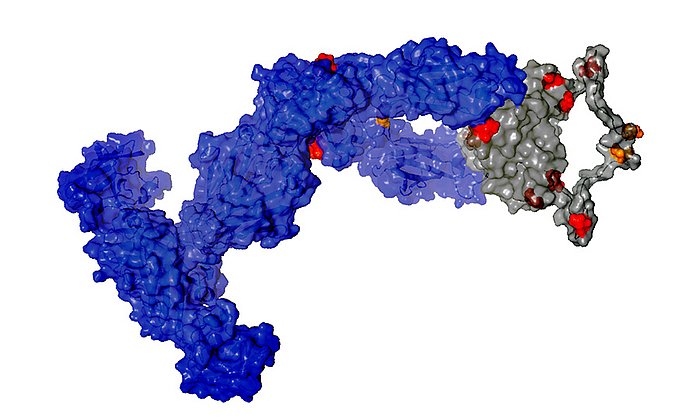
Antibodies are vital for the survival of human beings. They typically consist of two longer and thus heavier amino acid chains and two lighter ones. In rare cases, the plasma cells multiply excessively, flooding the body with light antibody chains.
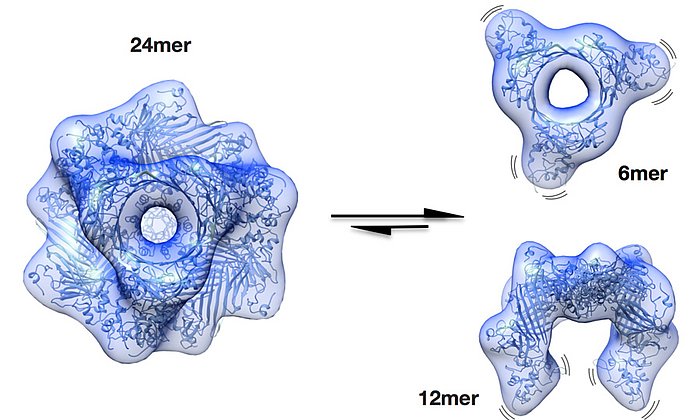
In people suffering from light chain amyloidosis (AL amyloidosis), these light chains are deposited as extremely fine fibers, so-called amyloid fibrils, in tissue or in organs. The disease is often recognized only after the deposits already compromise the function of organs. In many cases AL amyloidosis is fatal.
"To date, little was known about the exact cause of this amyloidosis," says Johannes Buchner, professor of biotechnology at the Technical University of Munich. “Depending on the organ affected, the symptoms vary considerably. Furthermore, each patient produces different types of antibodies. The disease is thus difficult to diagnose at an early stage.”
A mutation triggers the deadly disease
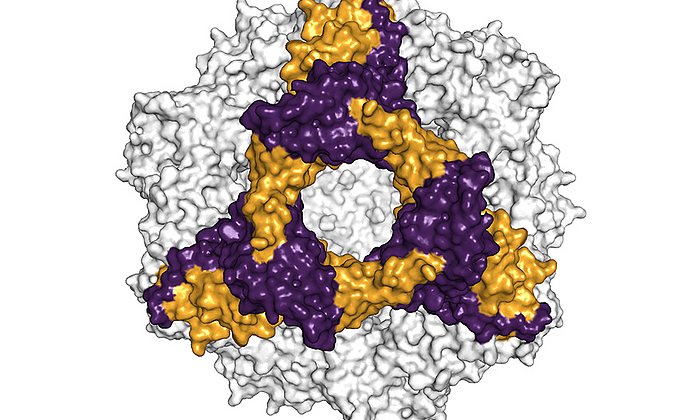
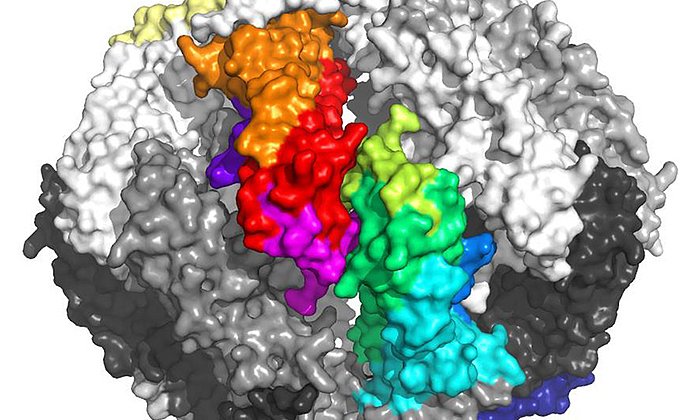
Using various analytical and database-supported methods, the team of scientists succeeded in identifying eleven mutations caused by the disease in the antibodies of a patient with advanced AL amyloidosis.
Further investigations showed that exactly one mutation was responsible for the destabilization and formation of the disease-causing amyloid fibrils. This mutation causes the unstable light chain to lose its structure after breaking into fragments, which then form the deadly amyloid fibrils.
"Our study shows that mutations that lead to unstable light chains are an important factor in the occurrence of amyloidosis," says Pamina Kazman, who carried out the majority of the measurements. "In the long term, we hope that these and other studies will lead to new, earlier diagnostic methods and possibly even new treatment options."
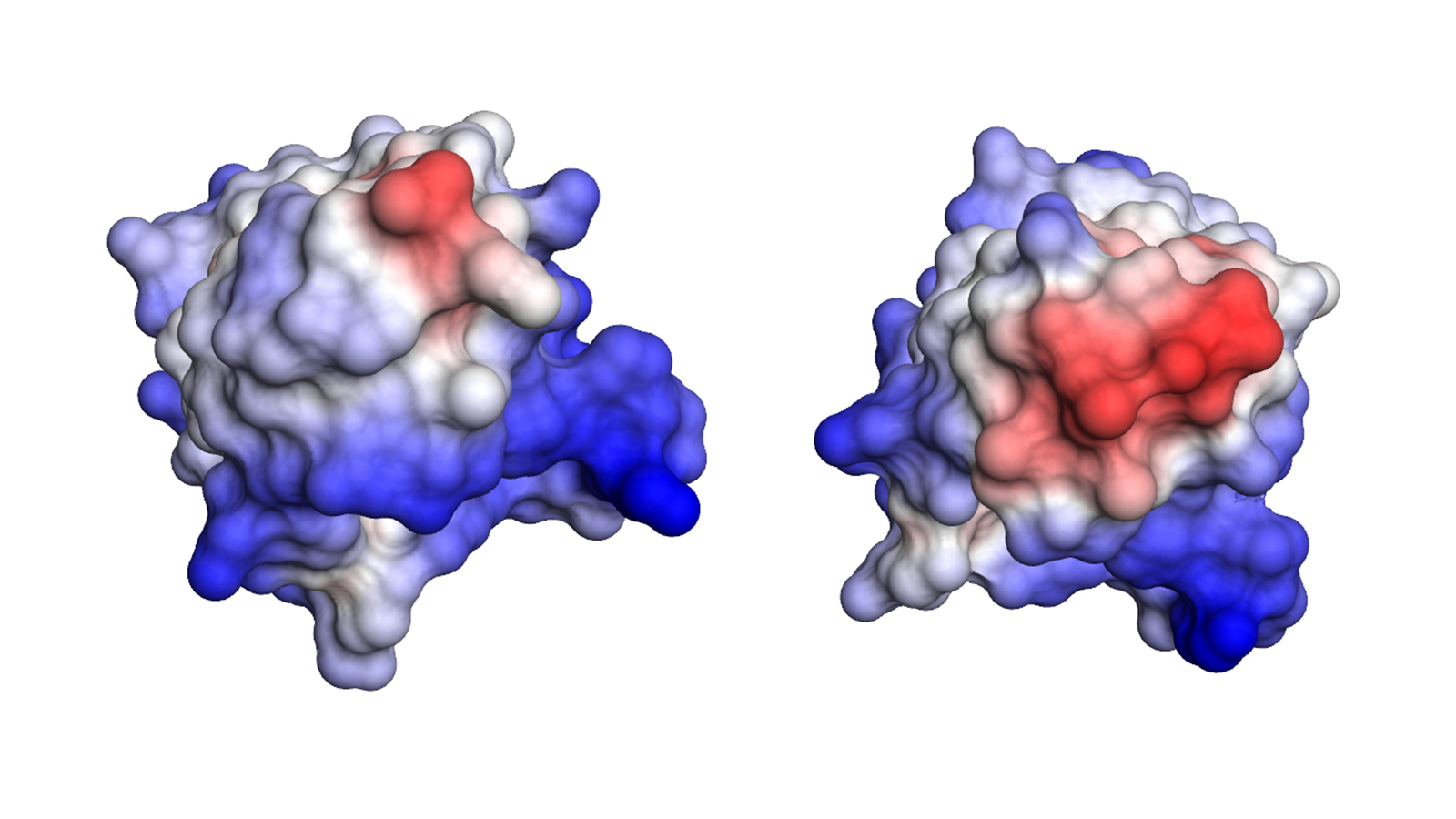
Fatal amyloid formation in a patient’s antibody light chain is caused by a single point mutation
Pamina Kazman, Marie-Theres Vielberg, María Daniela Pulido Cendales, Lioba Hunziger, Benedikt Weber, Ute Hegenbart, Martin Zacharias, Rolf Köhler, Stefan Schönland, Michael Groll, Johannes Buchner
eLife, online: 10.03.2020
The research was funded by the German Research Foundation (DFG). The protein structures were determined at the synchrotron radiation sources of the Paul Scherrer Institute in Villigen (Switzerland) and the European synchrotron radiation source in Grenoble (France).
Technical University of Munich
Corporate Communications Center
- Dr. Andreas Battenberg
- battenberg@zv.tum.de
- presse@tum.de
- Teamwebsite
Contacts to this article:
Prof. Dr. Johannes Buchner
Professorship of Biotechnology
Technical University of Munich
Lichtenbergstr. 4, 85748 Garching, Germany
Tel.: +49 89 289 13340 – johannes.buchner@tum.de
![[Translate to en:] [Translate to en:]](/fileadmin/_processed_/0/3/csm_p53_Figure_1600_053af93837.jpg)