Activation of heat shock protein explained:
Symmetrical protein activated by break in symmetry
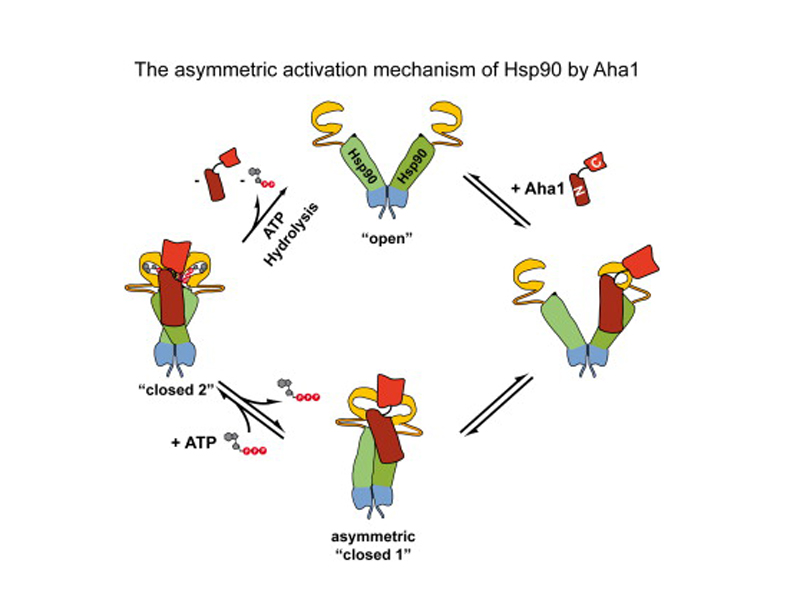
Every cell contains thousands of proteins. To control the life cycle of the cell, from division to death, the activities and lifespan of these proteins must be regulated. The heat shock protein Hsp90 plays a central role in this process. Hsp90 is a chaperone, a kind of “quality controller” that tests and monitors the quality and activity of numerous important signal proteins. If the cell is subject to a high level of stress, for example due to heat or oxygen deprivation, the production of this protein increases in order to limit the damage for other proteins.
The enormous complexity of the regulatory system of a cell is demonstrated by the fact that Hsp90 is also regulated by partner proteins. As evidenced by the example of cystic fibrosis, the disease also known as mucoviscidosis, this regulation is crucially important for the organism. It is known that this disease is reinforced considerably by the interaction between Hsp90 and a partner protein called Aha1. However, precisely how Aha1 influences the functioning of Hsp90 was unclear up to now.
Hsp90 is a protein composed of two identical symmetrical subunits. It was previously assumed that the two halves also work symmetrically, that is two Ah1 proteins are needed for its activation. At the TUM’s Department of Chemistry, a team of biochemists headed by Professor Johannes Buchner working in cooperation with Professor Horst Kessler’s group succeeded in explaining the mechanism for the activation of Hsp90 by its partner protein Aha1.
Surprisingly, the break in the symmetry of Hsp90 plays an important role in this process: The activation mechanism of Hsp90 works asymmetrically. The docking of an Aha 1 protein to two binding sites of an Hsp90 is sufficient to completely activate Hsp90. The researchers hope that in the long term the understanding of this mechanism could lead to the development of new approaches to the treatment of cystic fibrosis and other diseases, like cancer.
Publication:
Asymmetric Activation of the Hsp90 Dimer by Its Cochaperone Aha1
Marco Retzlaff, Franz Hagn, Lars Mitschke, Martin Hessling, Frederik Gugel, Horst Kessler Klaus Richter and Johannes Buchner
Molecular Cell 37, 344–354, February 12, 2010, DOI: 10.1016/j.molcel.2010.01.006
Contact:
Prof. Dr. Johannes Buchner
Technische Universität München
Department of Chemistry
Lichtenbergstraße 4 D-85748 Garching
Tel.: +49 89 289 13341 - Fax: +49 89 289 13345
E-Mail: Johannes.Buchner@ch.tum.de
http://www.biotech.ch.tum.de/