Neutrons help decipher key enzyme function
Facilitating processing of biomass
When producing synthetics, intermediate chemical products or biofuels from biomass, hemicellulose, a large polysaccharide abundant in plant cell walls, must first be broken down to monomeric sugars. In contrast to the uncatalyzed reaction in neutral solution, enzymes like glycosidases are able to speed up the decomposition reaction up to 18 orders of magnitude.
The biomass is typically pretreated in a very alkaline environment. However, natural enzymes develop their maximum activity in mildly acidic environments. An important research goal is thus to alter these enzymes in a manner to allow them to function effectively in a basic environment.
This requires detailed knowledge of the reaction sequence of the enzyme, in particular the precise position of all hydrogen atoms in the active center of an enzyme before, during and after the chemical reaction. This insight was lacking hitherto.
Detective work using neutrons
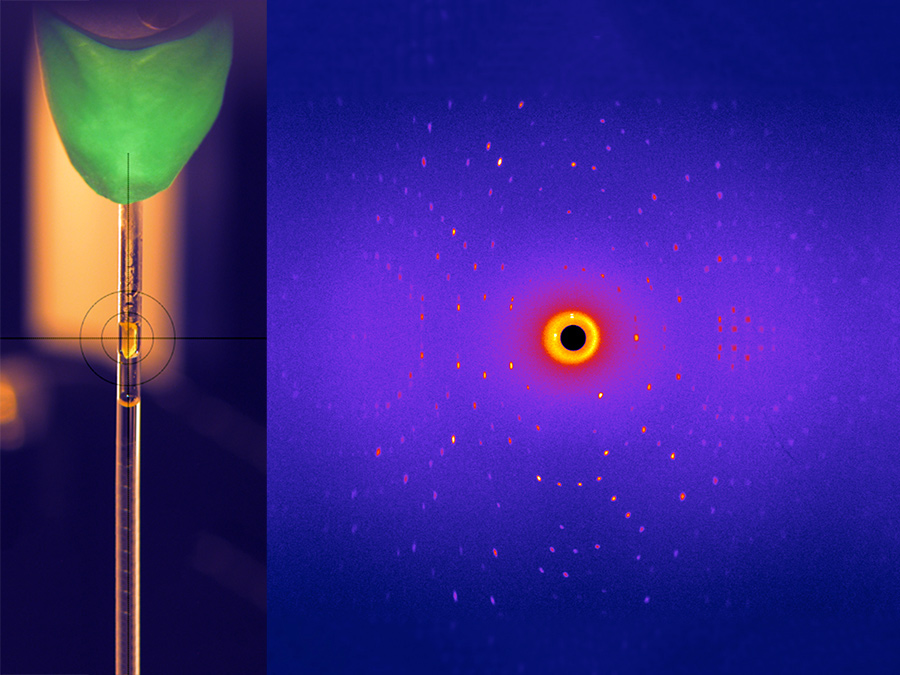
In the context of an international collaborative project, a team of researchers led by Andrey Kovalevsky, scientist at the Oak Ridge National Laboratory, has now determined the structure of a glycosidase with hitherto unprecedented accuracy. One of the three neutron sources used in the project was the research neutron source FRM II at TU Munich.
“Unlike X-ray structure analysis, neutron diffraction is the method of choice when it comes to pinpointing the position of hydrogen atoms in the active center of enzymes,” says TUM biologist Andreas Ostermann, who, in collaboration with his colleague Tobias Schrader from the Forschungszentrum Jülich, operates the BioDiff instrument in Garching at which the measurements were made.
The scientists analyzed the structure of the glycosidase using neutron scattering on enzyme crystals. They investigated the structure at various pH levels and in a complex with a ligand molecule. The insight gained from the neutron scattering experiments was used as a starting point for molecular dynamics simulations.
A turn for the better
During their work they discovered that the decisive step depends on the orientation of one specific amino acid side chain, the free carboxyl group of a glutamic acid. When it turns down and away from the substrate, it can take over a proton from a water molecule. When the carboxyl group turns upward, the acidity is strengthened and the proton is transferred to the substrate.
The lead author of the publication, Andrey Kovalevsky is very pleased with the success of the experiments: “No one has ever observed hydrogen atoms in a glycoside hydrolase enzyme, and until now we did not know how the catalytic glutamic acid residue is protonated.” Using this knowledge, work on altering the enzyme in a way that allows efficient biomass decomposition even in high pH environments can begin.
The research was funded by the Oak Ridge National Laboratory (ORNL), the Office of Basic Energy Sciences and the Office of Biological and Environmental Research of the US Department of Energy (DOE), the Chinese Ministry of Education and the Innovation Fund of the Yangzhou University. The measurements were carried out using the BioDiff instrument of the Heinz Maier-Leibnitz Zentrum (MLZ) at the research neutron source FRM II in Garching, as well as instruments at the neutron sources in Los Alamos und Oak Ridge and the Advanced Photon Source of the Argonne National Laboratory (USA). In addition, scientists from the Nanjing Agricultural University and the University of Toledo took part in the project.
Publication:
Q. Wan, J. M. Parks, B. L. Hanson, S. Z. Fisher, A. Ostermann, T. E. Schrader, D. E. Graham, L. Coates, P. Langan, and A. Kovalevsky; Direct determination of protonation states and visualization of hydrogen bonding in a glycoside hydrolase with neutron crystallography, PNAS 112, 12384 (2015) – DOI: 10.1073/pnas.1504986112
Contact:
Dr. Andreas Ostermann
Technical University of Munich
Research-Neutronsource Heinz Maier-Leibnitz
Lichtenbergstr. 1, 85747 Garching, Germany
Tel.: +49 89 289 14702 – E-mail – Web