Certain combinations of gut bacteria protect stem cell transplantation patients
The gut microbiome prevents dangerous immune reactions
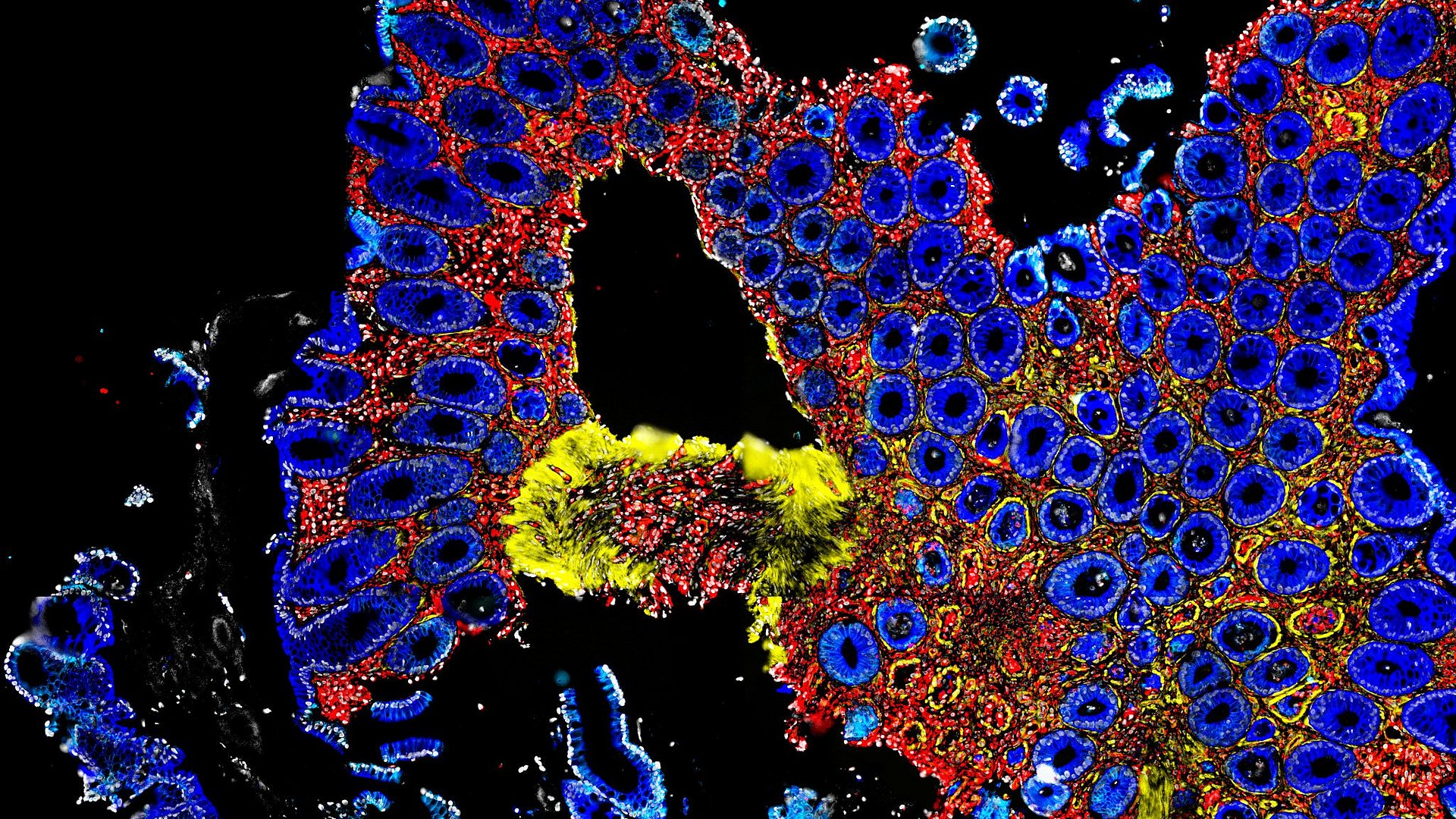
Stem cell transplantation can save the lives of patients suffering from cancers such as leukemia. However, graft versus host reactions occur following around half of these procedures. In a sense, they are the reverse of the rejection response seen after organ donations, in which the body attacks the donated organ. Here, the donated cells attack the patient’s body, for instance, in the digestive tract.
It has been known for some time that microbes in the gut play a role in determining whether GvHD occurs. A team working with Dr. Erik Thiele Orberg, who heads a research group at the Clinic and Polyclinic for Internal Medicine III of TUM’s Klinikum rechts der Isar, Ernst Holler, Senior Professor of Allogenic Stem Cell Transplantation at UKR, and Prof. Hendrik Poeck, executive senior physician at UKR’s Clinic and Polyclinic for Internal Medicine, describe in the journal Nature Cancer how the gut microbiome must be composed to provide protection.
78 patients observed
The researchers studied stool samples from 78 patients at the two university clinics and tracked them over two years following stem cell transplantation. They used the results to develop a risk index indicating the probability of a rejection reaction. “Instead of counting bacteria, we measured the quantities of certain metabolites produced by the microbes,” says Erik Thiele Orberg.
These immuno-modulatory microbial metabolites (IMMs) influence the immune system and the body’s regenerative capacity. “It is remarkable that a positive prognosis does not depend only on IMMs from bacteria,” says Dr. Elisabeth Meedt, a physician at UKR and co-first author of the article. “We demonstrated that certain viruses in the gut – the bacteriophages – also play a role. This alone offers an impressive insight into the complex world of our gut microbiome.”
Better prognosis with low microbiome scores
“Patients with a low IMM risk index had a higher chance of survival, showed fewer graft vs. host reactions, and experienced fewer relapses,” says Hendrik Poeck. The metabolites are formed mainly by bacteria from the families Lachnospiraceae and Oscillospiraceae in combination with the bacteriophages.
Actively improving the probability of recovery
In the next step, the researchers at TUM and UKR want to predict and actively improve patients' chances at a cure. “By precisely controlling the composition of fecal microbiota transplants, the gut could be colonized with specific consortia of bacteria and bacteriophages,” says Hendrik Poeck. “In the coming years, we want to find out whether we can use this approach to prevent graft vs. host reactions as well as relapses,” Initial experiments with mice have been successful. As a result, the procedure could now be tested in clinical trials with human patients.
E. Thiele Orberg, E. Meedt, A. Hiergeist, J. Xue, P. Heinrich, J. Ru, S. Ghimire, O. Miltiadous, S. Lindner, M. Tiefgraber, S. Göldel, T. Eismann, A. Schwarz, S. Göttert, S. Jarosch, K. Steiger, C. Schulz, M. Gigl, J.C. Fischer, K.-P. Janssen, M. Quante, S. Heidegger, P. Herhaus, M. Verbeek, J. Ruland, M. RM van den Brink, D. Weber, M. Edinger, D. Wolff, D.H. Busch, K. Kleigrewe, W. Herr, F. Bassermann, A. Gessner, L. Deng, E. Holler, H. Poeck. „Bacteria and Bacteriophage Consortia are Associated with Protective Intestinal Metabolites in Patients Receiving Stem Cell Transplantation”. Nature Cancer (2024). DOI: 10.1038/s43018-023-00669-x
- Virome expert Li Deng, Professor of Prevention of Bacterial Diseases at TUM, and Prof. André Gessner at the UKR Institute of Clinical Microbiology and Hygiene made substantial contributions to the study.
- Dr. Erik Thiele Orberg, Prof. Hendrik Poeck, Prof. Ernst Holler, Dr. Elisabeth Meedt, and Prof. Li Deng are members of the Collaborative Research Center (CRC) 1371 Microbiome Signatures, coordinated by TUM. Established in 2019, the center studies the interrelationships between the human digestive tract microbiome and diseases such as cancer and inflammatory bowel disease.
- Prof. Hendrik Poeck and Prof. Ernst Holler are members of the Collaborative Research Center TRR 221, which deals with unresolved challenges in the treatment of leukemia and lymphoma patients.
- Erik Thiele Orberg conducts research at TranslaTUM, the Central Institute for Translational Cancer Research at TUM, where doctors work with colleagues from the fields of natural sciences and engineering on research into causes, diagnostics and potential treatments of cancer. The goal is to rapidly translate new knowledge into patient care.
- For the project described in this release, Hendrik Poeck receives funding from an ERC Consolidator Grant as well as from the German Cancer Aid excellence program.
Technical University of Munich
Corporate Communications Center
- Paul Hellmich
- paul.hellmich@tum.de
- presse@tum.de
- Teamwebsite
Contacts to this article:
Dr. Erik Thiele Orberg
Technische Universität München
Klinikum rechts der Isar
Klinik und Poliklinik für Innere Medizin III
Tel. +49 89 4140 8066
e.orberg@tum.de
https://med3.mri.tum.de/de/forschung/nachwuchsgruppe-dr-erik-t-orberg
Prof. Dr. Hendrik Poeck
Universitätsklinikum Regensburg
Klinik und Poliklinik für Innere Medizin III (Hämatologie und Onkologie)
0941 944-5542
hendrik.poeck@ukr.de